Four Newer Antidepressants: Should You Use Them?
The Carlat Psychiatry Report, Volume 14, Number 4, April 2016
https://www.thecarlatreport.com/newsletter-issue/tcprv14n4/
Issue Links: Learning Objectives | Editorial Information | PDF of Issue
Topics: Antidepressants | Depressive Disorder | Practice Tools and Tips | Psychopharmacology Tips | Seasonal Affective Disorder
 Steve Balt, MD
Steve Balt, MD
Research fellow, Addiction Pharmacology Research Laboratory, California Pacific Medical Center
Dr. Balt discloses that his spouse is employed as a sales representative for Bristol Myers Squibb.
 Talia Puzantian, PharmD, BCPP
Talia Puzantian, PharmD, BCPP
Clinical psychopharmacology consultant in private practice, Los Angeles, CA.
Dr. Puzantian has disclosed that she has no relevant relationships or financial interests in any commercial company pertaining to this educational activity.
Since 2011, 3 new antidepressants have been approved by the FDA, and another (ketamine) has been generating buzz as a potential off-label medication for depression. In this article, we’ll take a step back and review the data on vilazodone (Viibryd), levomilnacipran (Fetzima), vortioxetine (Brintellix), and ketamine.
Vilazodone (Viibryd)
Vilazodone was approved by the FDA in January of 2011, making it the oldest of the newer antidepressants. Those who like tracking mechanisms of action are calling vilazodone a “SPARI,” which stands for serotonin partial agonist/reuptake inhibitor. The drug inhibits reuptake of serotonin (like SSRIs) and has partial agonism at 5-HT1A receptors (like buspirone). So, theoretically, giving your patients vilazodone is similar to giving them both an SSRI and buspirone at the same time. Is that a good thing? Nobody knows for sure. In the STAR*D trial, buspirone had a cameo appearance in one of the steps, being used as an augmenter of citalopram, and it worked as well as bupropion augmentation—a finding that may or may not have any relevance to vilazodone.
When the drug was first approved, the word on the street was that it (1) may work faster than other antidepressants, (2) may have fewer sexual side effects, and (3) may be more effective for anxiety. We were skeptical of these claims then, as was the FDA (see TCPR, April 2011 and this blog post). But new data have accumulated since then. We’ll rely mainly on a review published in 2015, which included 4 later-stage and post-marketing studies, as opposed to the pre-approval studies that the FDA reviews (Hellerstein DJ et al, Core Evid 2015;10:49–62).
Onset of action
The idea of faster onset of action was originally based on one piece of animal data and one piece of human data. The animal data showed that vilazodone quickly enhanced serotonin transmission in rats via 2 distinct mechanisms: 5-HT1A partial agonism and regular serotonin reuptake. In the human study, vilazodone showed statistically significant reduction in depression scores compared to placebo quite early, by week 1, although there was no active drug comparison (Rickels K et al, J Clin Psychiatry 2009;70(3):326–333).
Two more recent studies showed greater improvement versus placebo as early as week 2 (Croft HA et al, J Clin Psychiatry 2014;75(11):e1291–e1298; Mathews M et al, Int Clin Psychopharmacol 2015;30(2):67–74). However, antidepressant response at 2 weeks is not unique to vilazodone. Early improvement is the rule and not the exception for many antidepressants (Szegedi A et al, J Clin Psychiatry 2009;70(3):344–353). In addition, when researchers focused on remission instead of response, vilazodone took 6 full weeks to outperform placebo. The bottom line is that there is no convincing evidence that vilazodone has a faster onset of action than any of its competitors.
Sexual side effects
Early studies suggesting a cleaner sexual side effect profile for vilazodone were problematic. First, there was no SSRI comparator, which would have been necessary to make any claims that vilazodone had an advantage over other agents. Second, most of the patients enrolled had preexisting sexual dysfunction before being randomized to vilazodone or placebo. One can argue that this design has the advantage of being generalizable to many of our patients, who have underlying sexual dysfunction due to depression or age, for example. On the other hand, it’s akin to testing whether a drug has a headache side effect by giving it to a bunch of people who already had headaches. Any new-onset headaches would be obscured by the pathology already there. And indeed, in the company-funded study, treatment with vilazodone didn’t worsen the already high burden of sexual side effects—in fact, it was no different from placebo, both of which resulted in a slight improvement in sexual functioning (Rickels K et al, J Clin Psychiatry 2009;70(3):326–333).
In a more recent industry-funded post-hoc analysis of patients with normal baseline sexual function who were randomized to vilazodone, citalopram, or placebo, there were no significant differences in onset of new sexual side effects. The rates were: placebo: 12%; vilazodone 20 mg/day: 16%; vilazodone 40 mg/day: 15%; and citalopram 40 mg/day: 17% (Mathews MG et al, Abstract 45, ASCP 2014;). There was also no significant difference among those who had baseline sexual dysfunction: 33% of patients on placebo, 35% on vilazodone 20 mg/day, 30% on vilazodone 40 mg/day, and 28% on citalopram patients improved to normal sexual function by the end of the study.
According to the website ClinicalTrials.gov, there are ongoing studies of vilazodone addressing the sexual function issue. Until those results are published, we continue to consider the low sexual side effect claims as unsubstantiated.
Efficacy in anxiety
There’s a theoretical argument to be made that vilazodone’s 5-HT1A partial agonism might give it special anti-anxiety power. The only clinical trial evidence thus far is based on comparisons with placebo. As is true for many other antidepressants, vilazodone reduces scores on the Hamilton Anxiety Rating Scale more than placebo (Rickels K et al, J Clin Psychiatry 2009;70(3):326–333; Khan A et al, J Clin Psychiatr 2011;72(4):441–447). Another analysis of these data found that vilazodone may be more effective for the subgroup of anxious depressed patients than for the non-anxious depressed (Thase ME et al, Int Clin Psychopharmacol 2014;29(6):351–356). Promising, but we’d need data comparing this medication with other antidepressants to be convinced that it has an advantage.
TCPR Verdict: Based on this second look at vilazodone, we don’t see any new evidence that it works faster, has fewer sexual side effects, or is preferred in depressed patients with significant anxiety. We consider this a second-line antidepressant to be used after generics have failed.
Levomilnacipran (Fetzima)
Levomilnacipran was approved by the FDA in July 2013 for major depressive disorder. It is the close chemical cousin (an enantiomer) of milnacipran (Savella), approved in the U.S. in 2009 for fibromyalgia and approved for depression in other countries. Levomilnacipran is a serotonin and norepinephrine reuptake inhibitor (SNRI), which puts it in the same class as duloxetine (Cymbalta), venlafaxine (Effexor XR), and desvenlafaxine (Pristiq). However, levomilnacipran is more selective for inhibiting norepinephrine reuptake than the others—studies have shown that it has a 15-fold higher selectivity for norepinephrine than for serotonin. This selectivity disappears at higher doses.
But does norepinephrine selectivity mean anything clinically? Some researchers have hypothesized that there is a “norepinephrine deficit depression,” associated with poor concentration, inattention, low motivation, lack of energy, and cognitive impairment. This might be distinct from a “serotonin deficit depression,” more associated with anxiety, appetite disturbances, and suicidality (Moret C et al, Neuropsychiatr Dis Treat 2011;7Suppl1:9–13; Nutt DJ, J Clin Psychiatry 2008;69SupplE1:4–7). It would be nice if we could someday identify depressive subtypes that respond to specific medications, but the evidence for this norepinephrine/serotonin division is still indirect and preliminary.
Nonetheless, these speculations provide promotional talking points for reps, who may argue that their drug has a special norepinephrine-based power to improve impaired daily functioning.
Let’s look at the data.
Evidence on improving functioning
According to a recent meta-analysis, 4 out of 5 double-blind, placebo-controlled, short-term studies found that levomilnacipran was more effective than placebo for overall depressive symptoms (Montgomery SA et al, CNS Spectr 2014;5:1–9). The average response rate was 46% for levomilnacipran (vs. 36% on placebo) and the average remission rate was 28% (vs. 22% on placebo).
These studies also assessed change in functionality as a secondary measure. This was done using the Sheehan Disability Scale (SDS), a self-rating scale which asks about work/school, social life, and family life to measure functionality. Each of the three domains is scored from 0 (unimpaired) to 10 (extremely impaired). Any domain with a score of 5 or higher means significant functional impairment. So an SDS score of <12 total and <4 on all subscales indicates functional responders. An SDS score of <6 total and <2 on all subscales means functional remitters.
The meta-analysis reported a mean change in SDS score that was significantly greater with levomilnacipran compared to placebo but the actual difference in score was small, only a mean of 2.2 points better than placebo, (Sambunaris A et al, Int Clin Psychopharmacol 2014;29(4):197–205). The pooled response rate—that is, the percent of patients who functioned better at the end of the trial—was 39% for levomilnacipran vs. 29% on placebo, and the pooled remission rate was 22% vs. 15% on placebo.
Of course, the skeptic in us points out that any medication that eases depression is likely to also improve functioning. It may be that all antidepressants, regardless of their mechanisms of action, are just as effective as levomilnacipran for impaired functioning. Unfortunately, the company has not compared its drug with anything more robust than placebo, so we don’t know the answer yet.
An interesting secondary, post-hoc analysis of 1 of the 10-week placebo-controlled levomilnacipran studies looked at individual items in the major depression scales. The results didn’t support that levomilnacipran was better at any particular neurotransmitter profile of symptoms. Instead, the drug improved the same types of symptoms targeted by other antidepressants. So it’s unclear whether the higher selectivity for norepinephrine truly relates with any significant clinical outcome (Montgomery SA et al, Int Clin Psychopharmacol 2014;29(1):26–35).
TCPR Verdict: Levomilnacipran is an SNRI with especially strong reuptake inhibition of norepinephrine as opposed to serotonin. But whether it has any clear efficacy advantages over its competitors is not clear.
Vortioxetine (Brintellix)
Vortioxetine was approved by the FDA in September of 2013 for major depression. It’s considered a “multimodal agent,” meaning that it acts not only as a serotonin reuptake inhibitor but also affects several other serotonin receptors. It is an agonist of 5-HT1A receptors, a partial agonist at 5-HT1B receptors, and an antagonist at 5-HT3 and 5-HT7 receptors.
How well does vortioxetine work? A recent review of published and unpublished trials of the medication found 14 short-term randomized trials (6 to 12 weeks); eight of which were positive, five were negative, and one was considered “failed” because neither vortioxetine nor the active control, duloxetine, showed symptomatic improvement over placebo (Kelliny M et al, Ther Clin Risk Management 2015;11:1192–1212). Some studies compared vortioxetine to placebo, others to duloxetine or venlafaxine. Vortioxetine showed no clear superiority over active controls in measures of response or remission. So while vortioxetine has a distinctive pharmacological profile (Citrome L, Int J Clin Pract 2014;68(1):60–82), it is no more effective for core depressive symptoms than standard antidepressants.
The approved dose of vortioxetine is 10–20 mg/day. Sexual dysfunction has been reported to be minimal, but most premarketing trials relied solely on spontaneous reporting of adverse effects, which is known to underestimate their frequency (Cosgrove L et al, Account Res 2016 [Epub ahead of print]), and in one of the few trials that used a scale to measure effects on sexual performance, the authors concluded that “the sample number is too small to draw any conclusions” (Mahableshwarkar AR et al, J Clin Psychiatry 2015;76(5):583–591).
Is vortioxetine a smart pill?
As we know, “diminished ability to think or concentrate” is one of the DSM-5 criteria for major depression. Specific domains such as executive function, processing speed, attention, and learning and memory, have been found to be deficient during acute major depressive disorder (MDD) (Hammar A and Ardal G, Front Hum Neurosci 2009;3:26).
In an effort to get a leg up on its competitors, the manufacturer has done studies showing that vortioxetine improves patients’ performance on experimental cognitive tasks. Preclinical trials found that subjects on vortioxetine did better than those on duloxetine on the Digit Symbol Substitution Task (DSST), a measure of psychomotor speed (Gonzalez-Blanch C et al, Arch Clin Neuropsychol 2011;26(1):48–58). They then used the same outcome in 2 larger studies, each with 602 subjects. After 8 weeks, subjects on vortioxetine had higher scores on the DSST compared to those on placebo or those taking duloxetine, but by only 1.5%–3.0% (2 to 4 points on a 133-point scale) compared to placebo, and <0.5% (0.5 points) compared to duloxetine. On the strength of these studies, the company is applying for a new “cognitive dysfunction in MDD” indication. An FDA expert advisory panel recommended the approval in February, but just as we were sending this issue to press, the agency announced it would deny an expanded indication for cognitive dysfunction.
We assume that the FDA’s skepticism was related to a couple of important questions: First, do improvements on the DSST score translate into functional improvements that we (or our patients) would recognize clinically? Second, is vortioxetine any better than other antidepressants for improving cognition in depression?
In terms of the meaningfulness of its pro-cognitive properties, a recent meta-analysis found that while vortioxetine improves performance in the DSST, it didn’t help patients on 3 other cognitive tests. These include the Stroop test (a measure of cognitive control), the Trail-Making Test B (executive function), and the Rey Auditory Verbal Learning Test (delayed recall) (Rosenblat JD et al, Int J Neuropsychopharmacol 2015;19(2).pii: pyv082.doi:10.1093/ijnp/pyv082). As a smart pill, vortioxetine’s effects seem limited to one specific test—which doesn’t improve our confidence in its efficacy.
Finally, are the cognitive benefits of vortioxetine—however modest they may be—a direct pro-cognitive effect? Or do they indirectly follow from vortioxetine’s role as an antidepressant, thus implying that it won’t perform better than any other treatment that eases depression? This question has not yet been fully answered, although one manufacturer-sponsored trial claims that the higher DSST scores were independent of its antidepressant effect (Mahableshwarkar AR et al, Neuropsychopharm 2015;40(8):2025–2037). Similar claims have also been made for duloxetine (Greer TL et al, Dep Res Treat 2014. Published online 2014 Jan 19. doi: 10.1155/2014/627863), but other antidepressants simply haven’t been studied for their cognitive benefits.
TCPR Verdict: Will Brintellix make your patients “Brintellectuals”? The FDA is skeptical, and so are we.
Ketamine
Ketamine is not FDA approved for depression, but rather for preoperative general anesthesia. And it doesn’t act on serotonin, norepinephrine, or dopamine; instead, it’s an antagonist of the NMDA subtype of the glutamate receptor. It has long had illicit popularity in the party and rave scene under the nickname “special K.” Of relevance to psychiatrists, ketamine has been touted as a potential fast-acting miracle antidepressant, and many clinicians are already offering it off-label to their patients in pop-up ketamine clinics. Should you jump on the ketamine bandwagon?
The ketamine antidepressant data
As of late 2015, nearly a dozen randomized clinical trials of intravenous ketamine for the treatment of depression had been published (DeWilde KE et al, Ann NY Acad Sci 2015;1345:47–58). These include some placebo-controlled trials, in addition to some open-label trials and a few trials with an active control (usually midazolam [Versed]). All showed, on average, a statistically significant response—defined as a 50% reduction in MADRS or Hamilton Rating Scale for Depression (HAM-D) symptom scores—within 24 hours. Response rates have ranged from 40% to 70%. Some studies used only a single dose, with an antidepressant effect lasting up to 72 hours (even longer in some studies), while others involved repeated IV administrations over 2 weeks. The typical ketamine dose was 0.5 mg/kg given over a 40-minute period—as opposed to the anesthetic dose, which ranges from 1.0–4.5 mg/kg IV usually given over one minute.
Other studies have found that single infusions reduce suicidal ideation at 4 and 24 hours post-infusion (Price RB et al, Biol Psychiatry 2009;66:522–526). Investigators are now trying to identify subgroups who are more likely to respond to ketamine. There aren’t enough data yet to predict response, but some potential positive indicators include a family history of alcoholism, comorbid anxiety, or an elevated body mass index (Niciu MJ et al, J Clin Psychiatry 2014;75:e417–423).
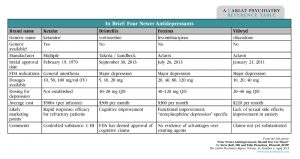
Table: In Brief: Four Newer Antidepressants
(Click here to view full-size PDF.)
Ketamine in the office?
So if it provides such rapid relief to some people who have been refractory to other treatments, why hasn’t ketamine caught on? One major hurdle, of course, is the fact that it’s an intravenous medication, making it much more complicated to prescribe than a pill. Because of potential, though rare, side effects such as an acute hypertensive crisis, the IV infusion should take place in a medical office equipped with vital sign monitoring, airway equipment, oxygen, and a crash cart. Some even advise the presence of a trained anesthesiologist (Sisti D et al, Curr Psychiatry Rep 2014;16:527). These requirements likely explain the high out-of-pocket costs (up to $500–$750 per infusion) for this off-label procedure at the handful of ketamine clinics that have popped up nationwide over the last few years. Other potential adverse effects, like an uncomfortable dissociative experience, as well as long-term cognitive impairment and the risk of diversion or recreational abuse of ketamine, must be considered.
Furthermore, no one really knows how long to provide the treatment. In the 2-week trials described above, which involved 6 infusions, relapse rates were as high as 55% to 89% in the month following treatment (Newport DJ et al, Am J Psychiatry 2015;172:950–966). No “maintenance” strategy has been described, and no other medications have been shown to extend ketamine’s antidepressant effect.
Finally, it’s still not clear that the standard 0.5 mg/kg intravenous dose is the “best” dose. This dose was chosen, in part, because it produces few side effects; these are typically transient dissociative symptoms (“I feel like I’m floating”) or hallucinations during the infusion. While these effects are short-lived, they have also been positively associated with a treatment response (Luckenbaugh DA et al, J Affect Disord 2014;159:56–61). Thus, dissociative effects may predict—or may even be responsible for—the antidepressant effect. If this is true, it may be hard to find a dose that minimizes unpleasant psychoactive effects while also producing a robust antidepressant effect. Then again, some practitioners are deliberately using higher doses of ketamine, sometimes in intramuscular or oral forms, in order to induce a psychedelic state, which they see as a necessary component of healing (Dakwar E et al, Drug Alc Depend 2014;136:153–157).
Pharmaceutical companies have eagerly embraced the ketamine story, in hopes of developing a similar drug without ketamine’s reputation and its pesky DEA Schedule III designation. But the options are limited. AstraZeneca tested one compound, lanicemine, but quietly backed out after it failed a Phase IIb trial in 2015. Another compound called GLYX-13 (recently renamed rapastinel), a partial agonist at another site on the NMDA receptor, has been effective in reducing HAM-D scores relative to placebo at some doses, and further research is ongoing. Other labs are studying the tuberculosis drug D-cycloserine, another NMDA modulator, as well as other agents. The closest thing to ketamine in the commercial pipeline is Janssen’s intranasal S-ketamine (an enantiomer of ketamine), currently in phase II trials.
Of course, if you want to explore this territory on your own, IV ketamine is readily available. It can be compounded into oral, sublingual, and intranasal forms. But its use in depression remains strictly off-label and, at this time, must be seen as experimental. As more data become available and protocols are published and refined, it may be worth your time and effort to add it to your repertoire.
TCPR Verdict: Ketamine looks promising for extremely rapid relief of depression—but the effects are short-lived, and any antidepressant that requires a crash cart nearby is not likely to become a blockbuster.