A Second Look at Genetic Testing
The Carlat Psychiatry Report, Volume 17, Number 3, March 2019
https://www.thecarlatreport.com/newsletter-issue/tcprv17n3/
Issue Links: Learning Objectives | Editorial Information | PDF of Issue
Topics: Free Articles | GeneSight | Genetic Testing | Pharmacology
 Chris Aiken, MD.
Chris Aiken, MD.
Editor-in-Chief of The Carlat Psychiatry Report. Practicing psychiatrist, Winston-Salem, NC.
Dr. Aiken has disclosed that he has no relevant financial or other interests in any commercial companies pertaining to this educational activity.
Genetic tests are marketed with a bold claim: that a handful of genes can predict medication response. AssureRx’s GeneSight is the most popular of these tests, and in 2015 we reviewed the evidence behind its panel. In short, it was lacking, but the company has just released new data that may move it a little closer to the mainstream.
What’s in the test
GeneSight looks at two areas where genetics can influence medication response:
- Pharmacokinetics. Around 20%–30% of people have genetic variations in their metabolic enzymes that can lead to levels of many psychiatric medications that are abnormally high (ie, slow metabolizers) or abnormally low (ie, fast metabolizers).
- Pharmacodynamics. While pharmacokinetics is about serum levels, pharmacodynamics is about the brain’s response to medications, which may be shaped by genetics.
GeneSight uses this information to create a stoplight with three categories of recommendations: Green (no worries—go ahead and prescribe this medication), Yellow (proceed with caution), and Red (best to avoid). Other companies present their results in a similar style, but each company’s results are driven by different, proprietary algorithms, which limits the ability of independent investigators to evaluate their validity. In other words, we’re stuck with industry-supported trials, and so far GeneSight has the largest number of them.
What we know so far
Before this new study was released, GeneSight’s clinical data had run into a brick wall. The positive studies were poorly designed, while the well-designed studies were negative. Two open-label, non-randomized studies with a total of 209 subjects suggested that GeneSight improved outcomes in depression. However, a randomized, double-blind trial failed to replicate that benefit (Zeier Z et al, Am J Psychiatry 2018;175(9):873–886). That negative study was small, involving 49 subjects, so the company undertook a larger study to see if they could demonstrate the value of their test.
A new study
GeneSight’s latest study is the largest to date, involving 1,167 subjects with moderate to severe treatment-resistant depression. They were randomized to receive antidepressant therapy that was guided either by GeneSight or by the clinician’s usual care. Most patients had failed at least 3 antidepressants, although only 1 failure was required for study inclusion. After 8 weeks, there were no significant differences between the two groups on the primary outcome measure, which was improvement on the Hamilton Depression Rating Scale (HAM-D). Although the study failed on the primary measure, results were marginally positive on secondary outcomes of response (≥ 50% improvement on the HAM-D, achieved by 26% in the GeneSight group vs 20% in the controls) and remission (final HAM-D ≤ 7, achieved by 15% in the GeneSight group vs 10% in the controls) (Greden JF et al, J Psychiatr Res 2019;111:59–67).
How impressive are these results?
A glance at GeneSight’s new brochure would have you believe the results are quite impressive. The secondary outcomes are on the cover, magnified to accentuate a small difference. In reality, you’d need to test 20 patients with treatment-resistant depression to bring 1 patient to remission. The results look a little better when limited to those patients who were switched from medications that were poorly matched with their genes to those that, according to the test, were a better fit. In this group, you’d need to test 8 patients to bring 1 to remission. That’s still fairly modest, and keep in mind that only 1 in 5 patients entered the study on medications that did not match their genes.
A bigger problem is that the primary measure was negative, and in a large study like this, such a failure is fairly definitive. Secondary measures are meant to explore possibilities, not confirm the truth, because every time one is added, it increases the chance of a false positive. No one should boast about secondary measures, and these aren’t even much to boast about.
Also, the study was not truly double-blind, a flaw shared by all the controlled trials in this field. Patients and raters were blinded to the presence of genetic testing, but the doctors who chose the antidepressants were not. It’s possible that these physicians conveyed a little more enthusiasm about their choices when they knew those choices were guided by a genetic test.
Other contenders
GeneSight is one of over 40 commercially available tests. Three of their competitors have randomized controlled trials: NeuroIDGenetix, Neuropharmagen, and GeneCept.
NeuroIDGenetix uses a genetic panel similar to GeneSight’s and just released a 12-week double-blind randomized controlled trial in 685 patients with depression or anxiety. Their paper boasts positive results, but they selectively reported the data, including only half of the sample (those with moderate to severe depression) in their final results (Bradley P et al, J Psychiatr Res 2018;96:100–107).
Neuropharmagen employs a much broader array of genes in their algorithm and recently released two major studies. As with GeneSight, the more rigorous the study, the less impressive the results. Neuropharmagen has two randomized controlled trials in depression, one from Korea (n = 100) and the other from Spain (n = 316). The Korean study was positive across the board, but it was single-blind; raters were not blinded to the use of the test. The Spanish study was double-blind, but like the GeneSight trial, it was positive only on secondary measures (Pérez V et al, BMC Psych 2017;17:250; Han C et al, Clin Psychopharmacol Neurosci 2018;16:469–480).
GeneCept takes a different approach, focusing on genes that regulate the transport of medications across the blood-brain barrier. If that transport is slow, higher doses may be needed. GeneCept’s panel only tells us about the dosing of medications, not their selection. However, it is the only test that has a positive, well-designed study without major flaws. In their 12-week randomized controlled trial of 148 patients with depression, the remission rate was 2.5 times greater in the gene-guided group: 28% vs 72% (Singh AB, Clin Psychopharmacol Neurosci 2015;13:150–156).
A few good tests
These clinical trials test proprietary algorithms, with results that are promising but not yet definitive. There’s another way to use genetic testing, though—you can skip the summary and look at the individual tests. Two groups, the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the FDA, keep a running tally of the drug-gene interactions that are valid and reliable. So far only a handful of pharmacokinetic genes have met their standards: CYP2C19, CYP2D6, and, with flibanserin (Addyi), CYP2C9. The groups have also weighed in on which medications are reliably altered by those genes, and they’re listed in the “Actionable Drug-Gene Interactions” table below. These are the drug-gene interactions worth paying attention to in a genetic panel.
Table: Actionable Drug-Gene Interactions
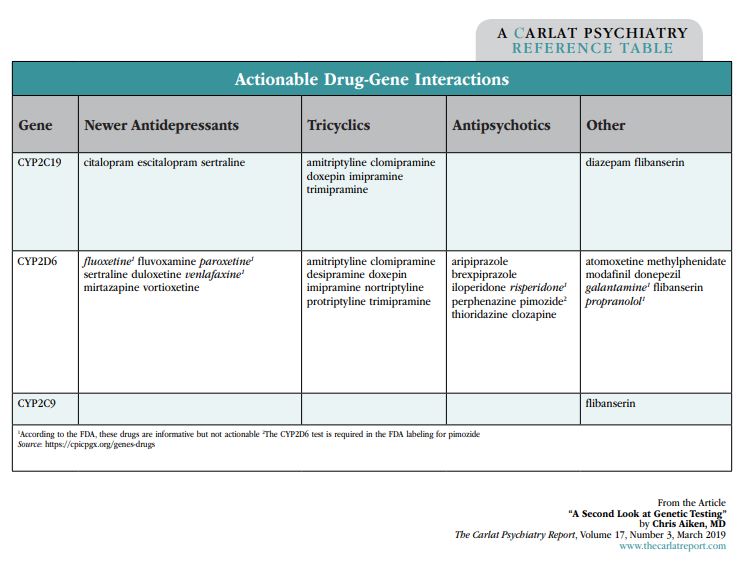
What about pharmacodynamic genes? The serotonin transporter gene is the most researched of these and is part of the GeneSight panel. Supposedly, the short version of this gene predicts lower response and more side effects with SSRIs, but the actual evidence on those points is mixed. The data on side effects did not hold up in a meta-analysis (Crawford AA et al, Eur Neuropsychopharmacol, 2013;23:1143–1150). For response to SSRIs, one meta-analysis found no relationship, while another concluded that the association was weak and only held up in European patients (Porcelli S et al, Eur Neuropsychopharmacol 2012;22:239–258; Taylor MJ et al, Biol Psychiatry 2010;68:536–543).
TCPR Verdict: Most genetic tests add a marginal benefit that is barely detectable in clinical trials. We don’t recommend them for routine use, but they may have a role in patients with moderate to severe depression who have failed multiple antidepressants. GeneCept has promising data to guide antidepressant dosing, but not selection, and that study needs confirmation. When interpreting genetic tests, take heed of the drug-gene interactions supported by good evidence, mainly the CYP2D6 and CYP2C19 genes.


