In Brief: Meds in the Fast Lane
The Carlat Psychiatry Report, Volume 19, Number 11&12, November 2021
https://www.thecarlatreport.com/newsletter-issue/tcprv19n11-12/
Issue Links: Learning Objectives | Editorial Information | PDF of Issue
Topics: Aducanumab | Cognition | Dementia | FDA | Neurology | Novel drug
Chris Aiken, MD.
Editor-in-Chief of TCPR. Practicing psychiatrist, Winston-Salem, NC.
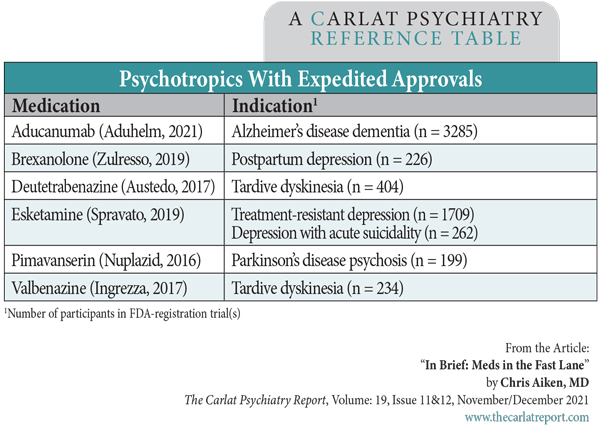
In the last five years, more psychotropics have gained approval through the FDA’s expedited pathways than the slow and cautious routes we’ve grown used to. These “fast-track” and “breakthrough therapy” approvals allow drugs to enter the market on the basis of more efficient (ie, smaller) clinical trials. Instead of the typical 2000 participants, many are approved through trials enrolling only 200.
For clinicians, this means the efficacy—and even more so the safety—of these expedited drugs cannot be fully trusted, particularly in the first few years of their release. Compared with traditional approvals, these drugs are 1.5 times more likely to see black box warnings added after their release (Mostaghim SR et al, BMJ 2017;358:j3837). In essence, our practices are the testing sites for the Phase III trials often skipped over in these approvals.
Both the fast-track and breakthrough designations require some initial evidence that the drug has the potential to fill an unmet need in a serious disorder. For that first step, the fast-track pathway allows animal studies, while the breakthrough pathway requires clinical data. In the end, both pathways require companies to provide clinical data to gain approval, but a third pathway, accelerated approval, allows biological markers to substitute for more direct clinical outcomes. For example, reduction in tumor size is a marker for cancer survival rates.
Aducanumab is the first neuropsychiatric drug to earn approval through the accelerated pathway, and the biomarker it depended on was amyloid plaques. However, it’s not clear that this is a meaningful marker of dementia, and that is just the beginning of the controversies surrounding this approval, which are detailed in this issue.
Biomarkers are unlikely to replace clinical outcomes in psychiatry anytime soon, but breakthrough and fast-track approvals are rapidly becoming the norm. Waiting in the wings are MDMA, psilocybin, an oral version of brexanolone (zuranolone), and combination pills for unipolar (bupropion-dextromethorphan) and bipolar depression (lurasidone-cycloserine). For patients with severe, refractory disorders, this is good news, and it’s for those patients that these meds should be reserved, at least until their safety is better established. Until then, we’ll need to keep our eyes wide open for unforeseen outcomes.
Table: Psychotropics With Expedited Approvals