Sex-Based Treatment of Schizophrenia
The Carlat Hospital Psychiatry Report, Volume 1, Number 5 & 6, July 2021
https://www.thecarlatreport.com/newsletter-issue/chprv1n5-6/
Issue Links: Learning Objectives | Editorial Information | PDF of Issue
Topics: Estrogen | Female Issues in Psychiatry | Gender | Hormone Replacement Therapy | HRT | Oral Contraceptives | Osteporosis | Post-menopausal | Prolactin | Raloxifene | SERM (Selective Estrogen Receptor Modulator) | Women’s Issues in Psychiatry
 Jayashri Kulkarni, MBBS, PhD, FAHMS
Jayashri Kulkarni, MBBS, PhD, FAHMS
Professor of Psychiatry, Monash University. Director, Monash Alfred Psychiatry Research Center, Melbourne, Australia
Dr. Kulkarni has disclosed no relevant financial or other interests in any commercial companies pertaining to this educational activity.
CHPR: Dr. Kulkarni, you’ve written about sex-based differences in symptoms and prognosis of schizophrenia. What are some of these differences?
Dr. Kulkarni: Compared to men, women with schizophrenia experience a more benign course of illness, including fewer psychiatric relapses and hospitalizations. They are more likely to be employed and married and to maintain interpersonal relationships. Also, women of reproductive age experience premenstrual exacerbations of schizophrenia that can lead to psychiatric hospitalization in the week prior to menses.
CHPR: Any differences in age of onset?
Dr. Kulkarni: Yes. A well-replicated finding is that the peak age of onset for men occurs between 18–24 years of age, while for women this peak is about 3–4 years later (Häfner H, Psychoneuroendocrinology 2003;28(Suppl 2):17–54). Women, but not men, have a second peak age of onset around 45–50 years, around menopause. Menopausal women, compared with younger women, display more refractory symptoms of schizophrenia. Animal studies provide another line of evidence showing that estrogen significantly impacts dopaminergic and serotonergic receptor systems, the main neurotransmitters implicated in the development of schizophrenia. These findings support the hypothesis that estrogen is a neuroprotective factor in this illness.
CHPR: How did the estrogen hypothesis influence your work?
Dr. Kulkarni: Our early studies were in uncharted territory. We had to find the dose and formulation of estrogen that would get into the brain and be helpful. We quickly stopped using oral estrogen because it doesn’t cross the blood-brain barrier and we instead used 17-beta-estradiol, which comes in a transdermal patch. We found the optimal dose was 100 mcg of transdermal estradiol. Then, around 2001, early evaluations of the Women’s Health Initiative (WHI) reported that the health risks of hormones—uterine and breast cancer, heart disease, and stroke—were greater than the benefits. This led to a sharp drop globally in prescriptions for hormone therapy. The data have since been re-analyzed, and it turns out that estrogen does have a benefit, but only when it is started early after menopause. It relieves menopausal symptoms, protects bones, and reduces the risk of colon cancer. There is a window of opportunity of about 10 years for beneficial effects, but beyond that, estrogen can be harmful.
CHPR: Where did this research lead?
Dr. Kulkarni: We then began working with selective estrogen receptor modulators (SERMs), which were a new class of medications that do not affect breast, uterine, or ovarian tissue. The first SERM, raloxifene, was developed to treat osteoporosis in menopausal women. We conducted the first pilot study of raloxifene in perimenopausal women with schizophrenia and found that at 120 mg daily, it was an effective adjunct in reducing total and general psychopathology (Kulkarni J et al, Psychoneuroendocrinology 2010;35(8):1142–1147). The subsequent randomized controlled trial confirmed a significantly greater reduction in the Positive and Negative Syndrome Scale (PANSS) total score (Kulkarni J et al, JAMA Psychiatry 2016;73(9):947–954).
CHPR: Is raloxifene well tolerated?
Dr. Kulkarni: The main side effects are hot flashes and leg cramping, but these tend to diminish over the first few months of treatment.
CHPR: Have other researchers found similar success with raloxifene?
Dr. Kulkarni: Yes, other groups have also found significant benefits for postmenopausal women taking adjunctive raloxifene. A recent meta-analysis summarized the findings and concluded that, at doses of 60–120 mg/day, raloxifene appears effective and safe (Zhu XM et al, Schizophr Res 2018;197:288–293).
CHPR: Do SERMs and estrogen benefit all symptoms of schizophrenia?
Dr. Kulkarni: 17-beta-estradiol appears to quickly reduce positive symptoms like hallucinations. Delusional and thought disorder symptoms improve a bit later, within the first week to 10 days. By about 2 weeks, we see cognitive improvements. With the SERMs, the positive symptoms also improve first. Some studies show that patients’ cognitive and negative symptoms also improve with SERMs, but not as much as with estradiol (Zhu et al, 2018).
CHPR: With what you know now about transdermal estradiol maybe not being as problematic as previously thought, would you recommend it over a SERM like raloxifene?
Dr. Kulkarni: It ends up as a case-by-case decision because you have to weigh the risks and benefits of the different options. In a woman who has a personal or family history of breast or uterine cancer, we would not want to prescribe estradiol. We also don’t want to use estradiol for women who smoke, or who have diabetes or hypertension. But estradiol might be a better choice than raloxifene for a menopausal patient with schizophrenia who is experiencing vasomotor symptoms like hot flashes.
CHPR: What type of screening do you complete prior to beginning raloxifene?
Dr. Kulkarni: We check for a past history of deep venous thrombosis or pulmonary embolism and measure factor V Leiden (a genetic mutation that increases the risk for blood clots) because raloxifene slightly increases the risk of deep venous thromboses and other thromboembolic events. These are rare side effects, with an absolute risk difference of 0.9 per 1000 woman-years (Martino S, Curr Med Res Opin 2005;21(9):1441–1452).
CHPR: Are you seeing psychiatrists prescribing raloxifene or estrogen?
Dr. Kulkarni: We are not seeing it. After more than three decades of research on the estrogen hypothesis, and multiple clinical trials finding that estrogen and SERMs are effective adjunctive treatments, I am surprised that this treatment approach has not been adopted into mainstream practice. Practice guidelines of physical health monitoring tend to focus on cardiovascular and metabolic disorders but overlook women’s health issues, like menopausal status or use of hormonal contraception. Also, specialists in general tend to have a “silo mentality” or disengagement from other areas of medicine—so in the case of psychiatrists, they might not be as open to learning about hormone treatments or contraceptive pills. It would be good to see a more holistic approach to treating patients with schizophrenia. Psychiatrists can work closely with their patients’ primary care clinicians to ensure that their female patients receive appropriate hormone treatments and have ongoing physical screening.
CHPR: You brought up an interesting point. Women with schizophrenia have relatively high numbers of unwanted pregnancies. Prescribing a contraceptive makes a lot of sense.
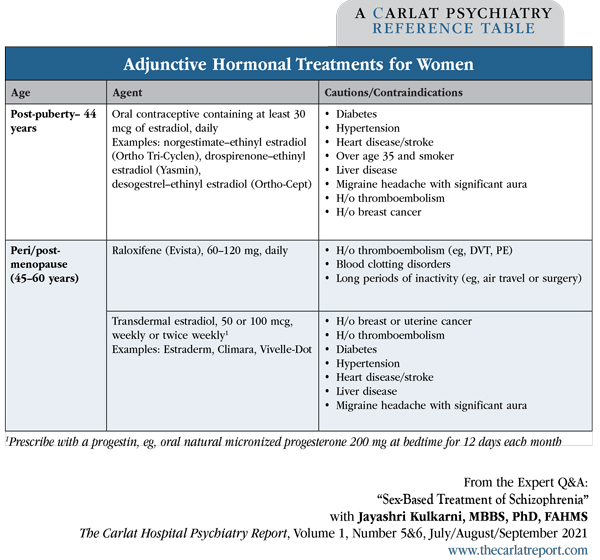
Dr. Kulkarni: Yes, we get two good outcomes with that single intervention. The oral contraceptive should preferably contain at least 30 mcg of estradiol (Editor’s note: See “Adjunctive Hormonal Treatments for Women” table below). For women with a uterus, the oral contraceptive should include a progestin to minimize the risk of uterine cancer. Third- or fourth-generation progestins, including norgestimate, drospirenone, desogestrel, and gestodene, are preferable because they are less likely to produce depressive side effects than first- and second-generation progestins like levonorgestrel and norethisterone (norethindrone), as was found in the Skovlund study (Skovlund CW et al, JAMA Psychiatry 2016;73(11):1154–1162). So we do use the oral contraceptive pills, but over time we have learned that all pills are not the same.
Table: Adjunctive Hormonal Treatments for Women
CHPR: How long do you continue raloxifene as an adjunctive treatment?
Dr. Kulkarni: Because the adjunctive use of raloxifene is new, I typically prescribe it for 2–3 years. We also want to make sure the patient is safe in terms of adverse events.
CHPR: Do you take any other considerations into account in your decision about whether to use or stop using raloxifene?
Dr. Kulkarni: We are not just looking at the benefit to psychotic symptoms, but also at the patient’s risk for bone loss, as raloxifene is FDA approved for the prevention and treatment of osteoporosis.
CHPR: That’s interesting, as rates of osteomalacia and osteoporosis among women with schizophrenia appear to be higher than for the general population of women.
Dr. Kulkarni: Absolutely. The hyperprolactinemic effects of antipsychotics suppress estrogen, and women who take antipsychotic medications are at greater risk for osteomalacia and osteoporosis. This is another example of “killing two birds with one stone,” where we can treat a woman’s psychiatric symptoms and help protect her bone health.
CHPR: Are there any other reasons we should worry about the bone health of our female patients?
Dr. Kulkarni: Women with schizophrenia often smoke and have poor nutrition, both of which increase the risk of bone density problems and potential fractures. There are several factors leading to poor bone health in women with schizophrenia.
CHPR: To summarize, it sounds like we should consider using estrogen or SERMs as adjunctive treatments for female patients, especially postmenopausal women who are not fully responding to antipsychotic medications and who are primarily experiencing positive symptoms.
Dr. Kulkarni: That’s right, but women of all ages can benefit from supplemental estrogen. For women of reproductive age, oral contraceptives can be helpful. For post-menopausal women, adjunctive raloxifene 120 mg is helpful. Alternatively, transdermal estradiol is another option and should be prescribed with a progestin if the patient has a uterus. Transdermal estradiol is particularly helpful for women with schizophrenia who also report hot flashes. These appear to be safe adjunctive treatments for schizophrenia.
CHPR: And if we do not feel comfortable prescribing these agents ourselves, we can work with our patients’ primary care physicians to help patients get started on these adjunctive treatments and get followed for any adverse sequelae.
Dr. Kulkarni: Yes, as long as patients are screened and monitored appropriately, raloxifene and transdermal estrogen appear to be safe adjunctive treatments for schizophrenia.
CHPR: Thank you very much, Dr. Kulkarni.